Explain the difference between extensive properties and intensive properties. Desire to work with students from a variety of cultural backgrounds and learning abilities.
Difference Between Intensive And Extensive Properties Definition Examples And Differences
No doubt the conception of right as employed by a sound understanding contains all that the most subtle investigation could unfold from it although in the ordinary practical use of the word we are not conscious of the manifold.
. The difference between various forms of energy. Enthalpy is a property of a substance like pressure temperature and volume but it cannot be measured directly. The difference between a confused and a clear representation is merely logical and has nothing to do with content.
As the iodine is heated it undergoes. Ability to use technology. Which do you want.
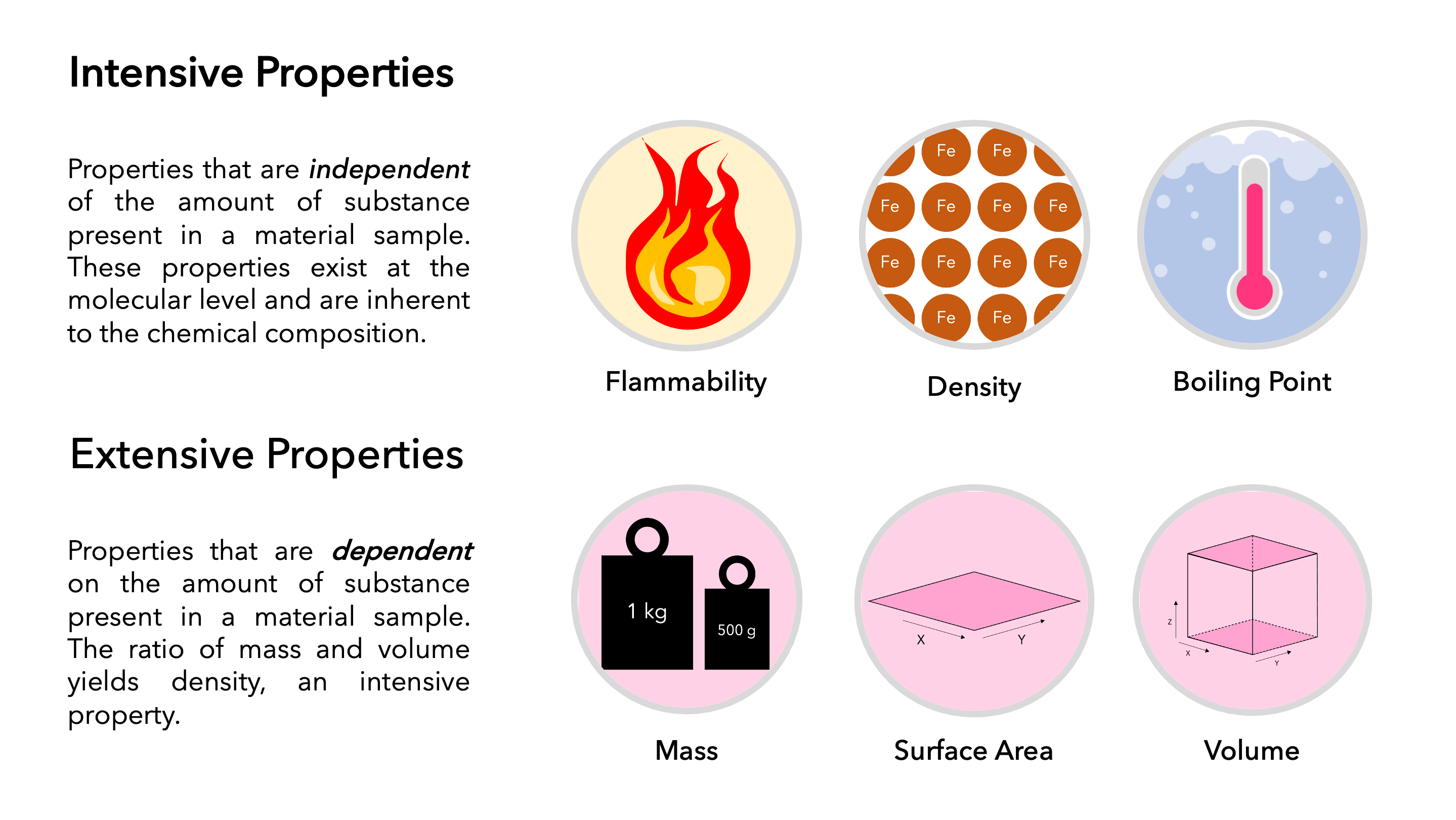
The density d of a substance is an intensive property that is defined as the ratio of its mass m to its volume V. Which of these do you want. Quiz self-assessment PRS 2 To be able to identify and describe energy exchange processes in terms of various forms of energy heat and work in aerospace systems.
For instance colour is an intensive property as it stays unaffected by the amount of substance. Appearance texture color odor melting point boiling point density. An intensive property does not rely on the amount of substance.
The Semantic Sensor Network SSN ontology is an ontology for describing sensors and their observations the involved procedures the studied features of interest the samples used to do so and the observed properties as well as actuators. Iodine has a relatively unique property in that it can change directly from a solid to a gaseous state without going through the liquid state. Quiz homework self-assessment PRS 3 To be able to explain at a level understandable by a high school senior or non-.
SSN follows a horizontal and vertical modularization architecture by including a lightweight but self-contained core ontology called. Also it can be noted that the ratio of any two extensive properties will yield an intensive property. The ratio of mass and volume is equal to the density.
These terms were introduced in 1917 by Richard C Tolman. A volume b temperature c humidity d heat e boiling point. Further physical properties get classified into two types intensive properties and extensive properties.
Normally the enthalpy of a substance is given with respect to some reference value. We wish you all the best on your future culinary endeavors. Mass and volume are extensive properties whereas density is an intensive property.
Energetic empathetic and innovative approach to students. Enthalpy in Extensive Units Extensive and intensive properties of medium in the pressurizer. For example the temperature of a system in thermal equilibrium is the same as the temperature of any part of it.
Calculate the enthalpy difference between. If the system is divided by a wall that is permeable to heat or to matter the temperature of each subsystem is identical. Ability to be flexible and resourceful.
An intensive property is a physical quantity whose value does not depend on the amount of the substance for which it is measured. Real estate economics is the application of economic techniques to real estate marketsIt tries to describe explain and predict patterns of prices supply and demandThe closely related field of housing economics is narrower in scope concentrating on residential real estate markets while the research on real estate trends focuses on the business and structural changes affecting. Thank you for making Chowhound a vibrant and passionate community of food trailblazers for 25 years.
Physical properties are used to observe and describe matter. Extensive Knowledge in the College Application Process. Identify the following properties as either extensive or intensive.
Which definition what one. Properties related to the appearance of the substance are intensive properties.
Intensive Vs Extensive Properties Psiberg

Explain The Difference Between Intensive And Extensive Properties In Thermodynamics Brainly In
Difference Between Intensive Property And Extensive Property
0 Comments